State of the Art Battery Technology for Automotive Application
A. Abstract
Owing to the detrimental impacts on the environment (greenhouse effect, global warming, etc.) and the depletion of existing energy resources, the automobile industry using Internal Combustion Engines is now under tremendous pressure. Hence, electrification of current transportation is the need of the hour. The major benefit of Electric Vehicles (EVs) is the contribution that they can make towards improving air quality in towns and cities. In this newsletter, an insight into the state-of-the-art battery technology for the automotive sector is provided. Comparisons between Li-ion Batteries (LIBs) and Sodium-ion Batteries (SIBs) are clearly illustrated for automotive applications. It also discusses various energy storage devices available in the market and their shortcomings.
B. Introduction
The transportation sector is one of the largest contributors to greenhouse gases as per the report of the United States Environmental Protection Agency (EPA) as shown in Fig.1.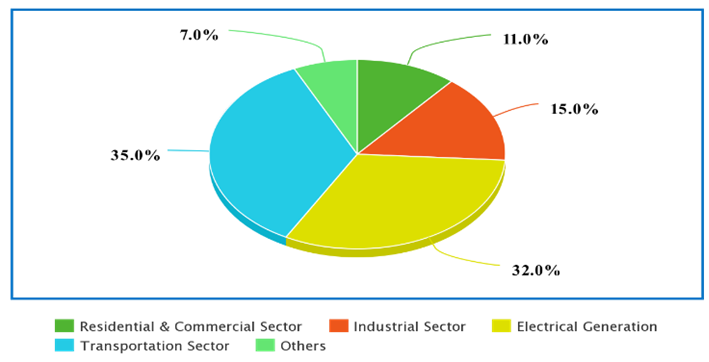
Fig. 1: CO2 emission by various sources [1]
The Global Fuel Economy Initiative (GFEI) aims to de-carbonize road transport and bring a targeted reduction of 65% in CO2worldwide by 2050. Therefore, the replacement of conventional vehicles with their electrical alternatives has gained momentum. By utilizing different technological aspects from multiple fields of engineering, the overall fuel consumption and efficiency of electric/hybrid electric vehicles (E/HEVs) are reduced making them more efficient than conventional vehicles. A battery pack, a power converter, and a mechanical transportation system assist in providing power to the electric motor for propulsion.
The energy density (Wh/kg), power density (W/kg), cycle efficiency, self-dis/charge characteristics, and life cycles are considered for the selection of energy storage devices (ESDs).
The most promising battery technology currently in use is LIBs because it has one of the highest energy densities (100–265Wh/kg or 250–670Wh/L). It delivers up to 3.6V which is three times more than technologies such as Nickel Cadmium (Ni-Cd) or Nickel-Metal Hydride (Ni-MH). LIB has no memory effect, meaning repeated partial dis/charge cycles could cause a battery to ‘remember’ a lower capacity and its self-discharge rate, 1.5–2% per month, is low. They are easier to dispose of than Nickel-Cadmium batteries as they release no toxic cadmium.
Though this technology is rapidly progressing there are a few notable disadvantages that include thermal instability, aging mechanism, and additional cost for protection [2].LIBs need a safety mechanism to limit voltage and internal pressure, which in turn increases their weight and limits their performance. They are also subjected to aging as they can lose capacity and frequently fail after a few years.
In order to minimize/overcome these issues, Sodium-ion Batteries (SIBs) came to light. As per research conducted at the Dept. of Energy’s Advanced Research Projects Agency, California, the cost of SIBs would be 10–20% lesser than LIBs. Sodium (Na) is more abundantly available than Lithium (Li) in the earth’s crust; therefore, it incurs a lesser cost for extraction and purification. Na cells possess a wider temperature range and are stable. They are inflammable and possess no thermal runaway. In addition, the cost of SI cells would be lesser for stationary applications like renewable energy storage for homes and grid or backup power for data centers, where cost is more important than size and energy density. The anode and cathode materials used in SI cells are Carbon and Sodium Metal Oxide. These materials are available in large numbers like Cobalt (cathode in Li-ion) which is toxic and has limited quantity. However, SIBs do have a few disadvantages in terms of performance for e-mobility and portable electronics. LIBs possess higher energy density than SIBs. To date, SIBs have demonstrated about half the energy density of LIBs, about 285Wh/kg [3].
Tables 1 and 2 show a comparison between different types of cathode material used in LIBs and SIBs [4]. Here, working voltage is the voltage specified by the manufacturer at defined atmospheric conditions considering safety constraints. The initial capacity is the amount of active material present in the cell during the time of manufacturing. It also indicates the maximum amount of energy that can be drawn from the battery under specified operating conditions. The cycling stability of a battery is defined by the number of dis/charge cycles until its capacity is reduced to a certain amount of its nominal capacity. 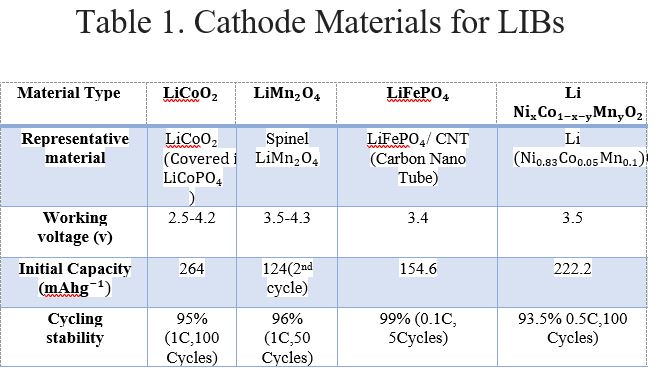
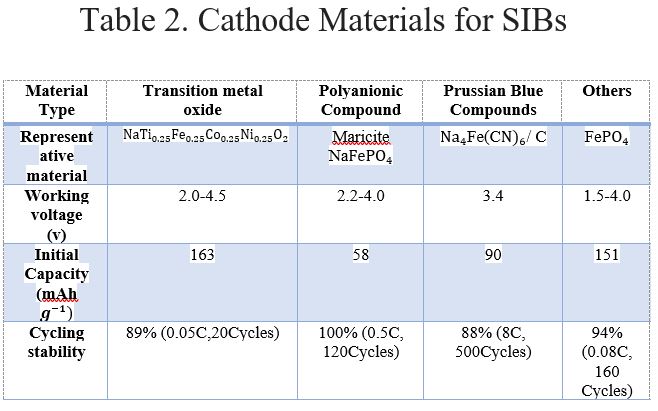
It is observed that the approximate working voltage for Li cells and Na cells is 3.5Vand 2V, respectively. Therefore, the working voltage of LIB > SIB. Hence, higher power can be extracted. The specific density of LIB > SIB and LIBs have a longer cycle life. Solid oxide fuel cells (SOFCs) are gaining popularity and are emerging energy sources for E/HEVs. The cathode material composition largely affects the performance of SOFCs. One of its biggest degrading factors is cathode poisoning when alloys composed mainly of chromium are used as the interconnect material. Therefore, it is imperative to develop chromium-tolerant cathodes. For SOFCs operating at high-temperature ranges, specifically at 800–1000 °C, the suitable cathode material is a composite of Sr-doped LaMnO3 (LSM) and yttria-stabilized zirconia (YSZ). There are also some mixed ionic-electronic conductors (MIECs) which are used as the cathode.
C. ESD for automotive applications
Energy storage subsystems play a significant role in E/HEVs as they power both the auxiliary and the propulsion subsystems. They are responsible for the control and regulation of energy flow in the EV. Its important characteristics include storage capacity, energy density, power density and high cycle life. Fig. 2 illustrates the different ESDs used in automotive applications.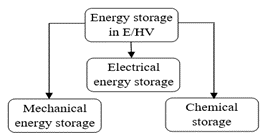
Fig. 2. Energy storage technologies in E/HV
For electrical energy storage, batteries and Ultra Capacitors (UCs) are primarily used owing to their reliability, technology maturity and ease of availability. Comparatively, batteries have high energy density (30–150 Wh/kg), moderate power (<0.5 kW/kg at 95% efficiency), short shelf life (1-year w/o recharge), and short cycle life (1Kcycles, deep discharge). Their cycle life and power characteristics are dependent on the State of Charge (SOC). While UCs have low energy density (<10 Wh/kg), longer shelf life (>10 years), and cycle life (>100Kcycles), their cycle life and power characteristics are independent of SOC.
Mechanical energy storage primarily comprises flywheels, hydraulics, and compressed air systems (CAES). On a commercial scale, pumped hydro and flywheels are the most popular. However, they are less preferred for automotive applications as compared to other storage technologies. The capacity and power of the flywheel fall in between those of UCs and batteries. They are designed for vehicles with a power density of 5.5 kW/kg and an energy density of 3.5 Wh/kg. Comparatively, they have a slightly longer discharge time than electric storage systems. CAES have lower capacities than pumped storage systems when duration greater than 24 hours is considered.
Chemical energy storage is used in HEVs where liquid fuel like gasoline along with small batteries (regenerative braking charges the battery using energy from wheels) is used. Gasoline has a very high energy density (~50–100times) when compared to LIBs. However, this shortcoming in LIBs is partly compensated by an electric motor (having a very high efficiency of ~60–80 %) during energy conversion from the battery to the propulsion system. Hydrogen (H2) fuel cells are gaining popularity. Although H2 is comparatively very light (energy density greater than that of liquid fuels), uncompressed H2 has disadvantages. Due to its bulkiness, it is practically difficult to accommodate in a vehicle. To compress H2, high-pressure tanks are needed which again compromises the space in vehicles. Alternatively, Vanadium Redox Flow Batteries (RFBs) are also popular energy storage batteries used in large-scale utilities and in commercial and residential energy storage systems. However, materials used in RFBs like vanadium and electrolytes, need to be sourced carefully. The specific energy (25–35 Wh) of RFBs is also comparatively low. There are concerns regarding the packaging and safety of RFBs for vehicle applications.
D. Conclusion
ESDs can be mainly classified into electrical, mechanical, and chemical. Batteries, FCs, and CAES are popularly used for automotive applications. However, batteries are best suited for automotive applications due to their high energy and power densities and low self-discharge rates. Though SIBs are cost-effective and do not possess thermal instability, they cannot be used to power EVs due to low storage and output power capability. These batteries outperform LIBs in applications related topower back to the grid and other stationary applications.
The authors declare no conflict of interest. No data/information from Bosch Global Software Technologies (BGSW) was used for this work.
REFERENCES
- United States Environmental Protection Agency (EPA) “Overview of Greenhouse Gases. Greenhouse Gas (GHG) Emissions (2020).” https://www.epa.gov/ghgemissions/overview-greenhouse-gases#carbon-dioxide (accessed Oct. 1, 2020).
- Sumukh Surya. “Thermal Runway in Lithium-Ion Battery for Mobility Applications.”IEEE TEC March 2022. https://tec.ieee.org/newsletter/march-2022 (accessed May. 1, 2022)
- Prachi Patel. “Sodium-ion Batteries Poised to Pick Off Large–Scale Lithium-ion Applications.”IEEE Spectrum 2021. https://spectrum.ieee.org/sodium-ion-battery (accessed May. 1, 2022)
- Tian, Wenchao, et al. “The Research progress and comparisons between Lithium-ion battery and Sodium-ion battery.” 2019 IEEE 19th International Conference on Nanotechnology (IEEE-NANO). IEEE, 2019.
Authors
 |
Sumukh Surya is currently a Senior Engineer at BGSW and his research interests include modeling of power electronic converters, electric energy storage systems, and the development of Battery Management System (BMS) algorithms for transportation electrification. |
 |
Dr. Mohan Krishna S is presently an Associate Professor at Alliance University, Bangalore, India. His research interests include electricvehicles, clean energy technologies and IoT-based building energy management systems, etc. |
 |
Ahilya Chhetri is a Technical Editor, Engineering and Technology at Cactus Communications. Her current research interest lies in power electronics, electric vehicles, building/space management systems, and Battery Management Systems (BMS). |
About the Newsletter
Editors-in-Chief

Jin-Woo Ahn
Co-Editor-in-Chief

Sheldon Williamson
Co-Editor-in-Chief
TEC Call for Articles 2023 - Advances in Charging Systems
The TEC eNewsletter is now being indexed by Google Scholar and peer-reviewed articles are being submitted to IEEE Xplore.
To submit an article click here.


